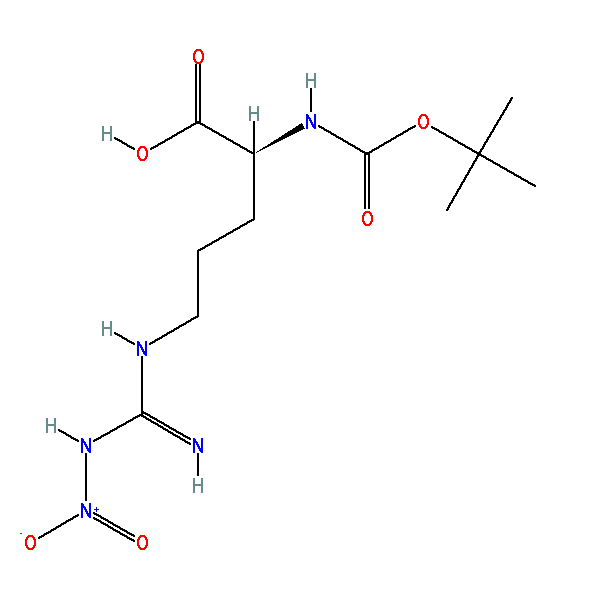
Rapid, effective deprotection of tert-butoxycarbonyl (Boc) amino acids and peptides at high temperatures using a thermally stable ionic liquid | Semantic Scholar

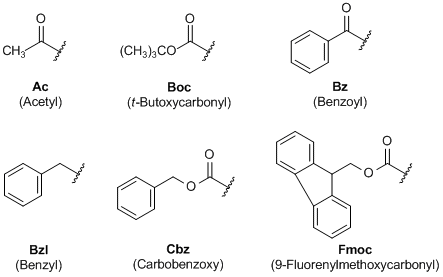
An efficient and highly chemoselective N-Boc protection of amines, amino acids, and peptides under heterogeneous conditions | SpringerLink

China Customized 4-(N-Boc-amino)phenylboronic Acid Pinacol Ester 330793-01-6 Suppliers, Manufacturers, Factory - ALLYCHEM
Boc-L-Threonine, 5 g, CAS No. 2592-18-9 | tert-Butyl / Boc | Amino acids, protected | Amino Acid Derivatives | Amino Acids and Amino Acid Derivatives | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International

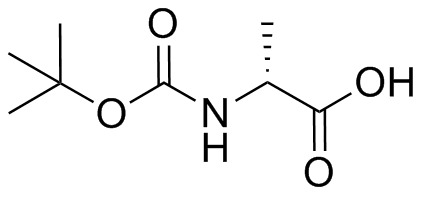
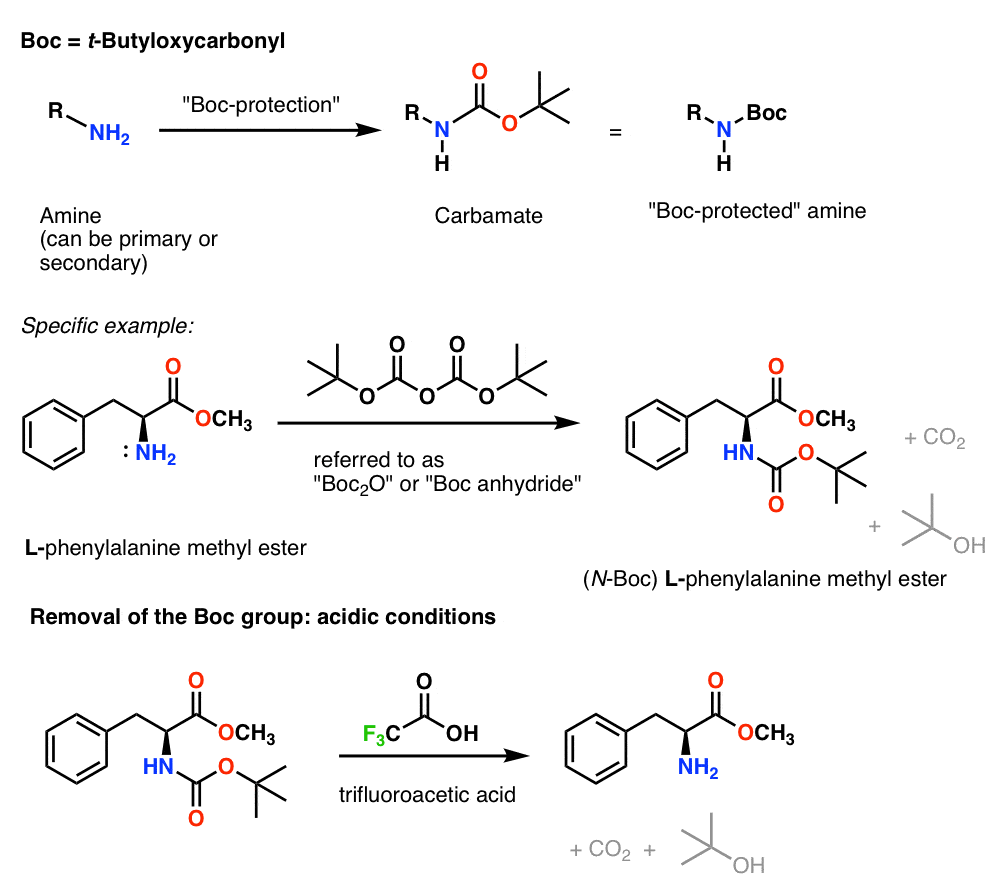
The BOC-protecting group may be added by treatment of an amino acid with di-tertbutyl dicarbonate as shown in the following reaction sequence. Propose a mechanism to account for formation of these products.

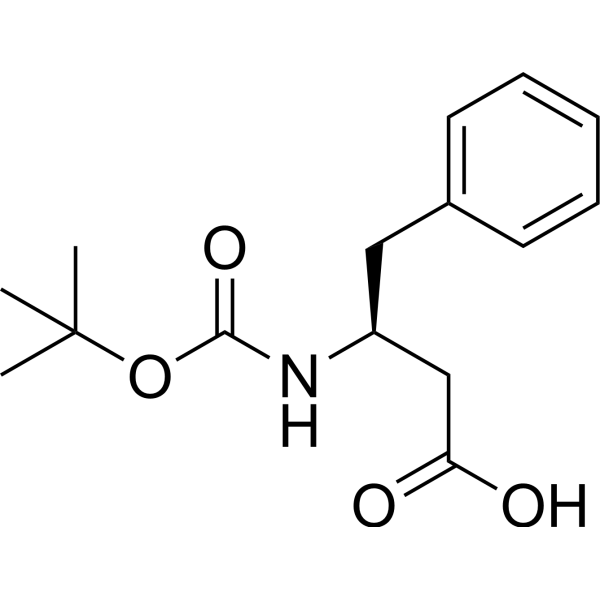
Direct amidations between N-Boc protected β-amino acid and C-protected... | Download Scientific Diagram

DES-catalyzed deprotection of N-Boc amino acid derivatives and N-Boc... | Download Scientific Diagram
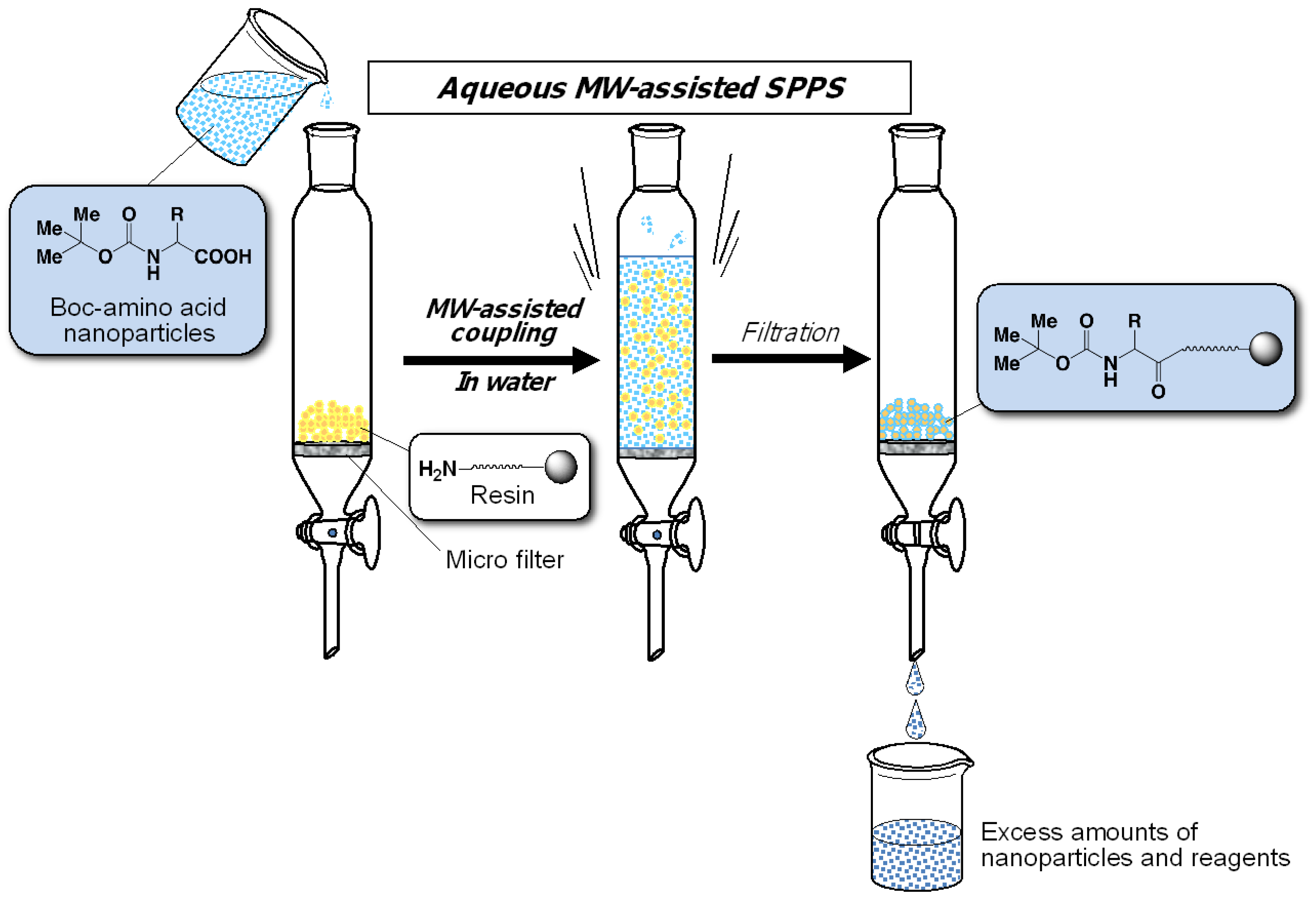
Applied Sciences | Free Full-Text | Aqueous Microwave-Assisted Solid-Phase Synthesis Using Boc-Amino Acid Nanoparticles

293302-31-5 (net) | Bis-Boc-amino-oxyacetic acid monohydrate | = 98% (HPLC) - Chem-Impex International
Boc-L-Tyrosine, 5 g, CAS No. 3978-80-1 | tert-Butyl / Boc | Amino acids, protected | Amino Acid Derivatives | Amino Acids and Amino Acid Derivatives | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International